Photographed by Ben Ritter. INFORMATION and FDA-approved Medication Guide.
 2020 Fda Regulations For Food Labeling Are You Compliant Labelcalc
2020 Fda Regulations For Food Labeling Are You Compliant Labelcalc
This article originally published on July 1 2020 was updated to reflect the correct and current number of comments that have been received by the FDA about this food labeling guidance.
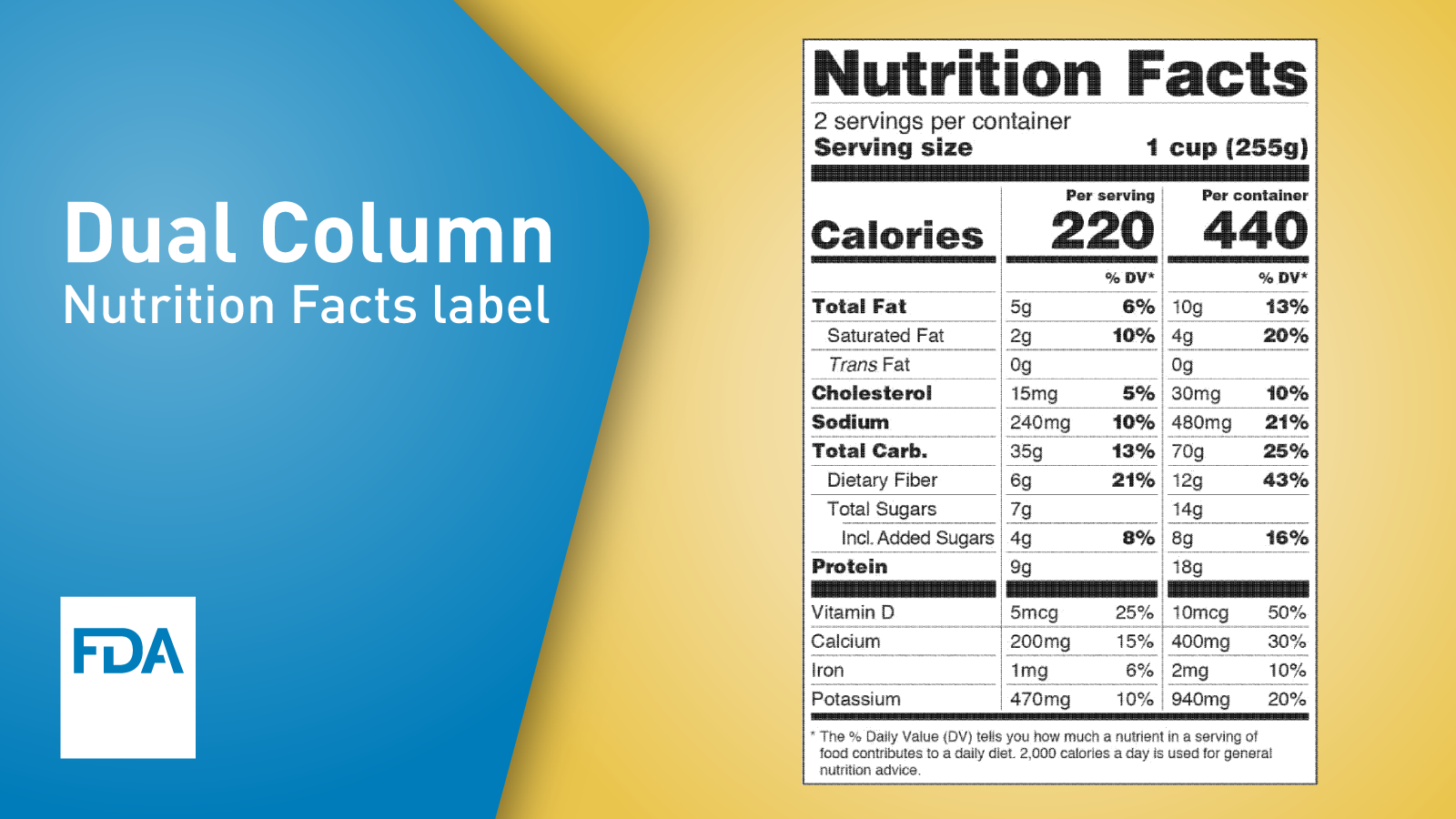
Fda food labeling guide 2020. The Use of an Alternate Name for Potassium Chloride in Food Labeling December 2020 Guidance for Industry. Basics of FDA Food Labeling Requirements Most food product labels have specific requirements about what information you must state on the label where it appears and the format. 11 Limitations of Use.
22 Concomitant Use with an Insulin Secretagogue eg Sulfonylurea or with Insulin. FDA When do these changes go into effect. FDA releases guide to help small food manufacturers meet nutrition labeling standards The US.
This guidance has been prepared by the Office of Nutrition and Food Labeling in the Center for Food Safety and Applied Nutrition at the US. Companies below that revenue mark or single supply manufacturers. 1 INDICATIONS AND USAGE.
Companies exceeding 10 million in revenue must comply with new changes by Jan 1 2020. CFR - Code of Federal Regulations Title 21. Prohibited introduction of misbranded foods drinks and drugs.
Food and Drug Administration. Revision of the Nutrition and Supplement Facts Labels. According to new FDA regulations regarding food labeling for food manufacturers.
FDA Seeks Input on Nutrition Labeling for Certain Sugars and Issues Final Guidance on Allulose. Final Rule on new Nutrition Facts Label. Labeling and Dietary Supplements Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park MD 20740-3835 Telephone.
The information on this page is current as of April 1 2020. The new food nutrition label. Large food manufacturers with more than 10 million in annual revenue were required to make the switch by Jan.
Prohibited color additives to conceal inferiorityuse of poisonous colors. FDAs New Nutrition Label goes into effect. Food Allergen Labeling and Consumer Protection Act.
FDA Seeks Input on Labeling of Food Made with Cultured Seafood Cells. Exemption from Nutrition Labeling Requirements. 2 DOSAGE AND ADMINISTRATION.
Office of Nutrition and Food Labeling HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park MD 20740 Tel 240-402-2373 Translations. The small entity compliance guide SECG is intended to help small entities comply with a final rule we issued in the Federal Register of May 27 2016 entitled Food Labeling. The Nutrition Facts label must list total fat saturated fat trans fat cholesterol sodium total carbohydrate dietary fiber total sugars added sugars protein and certain vitamins and.
Both the FDA and Health Canada have recently introduced new and differing Nutrition Facts Table regulations adding to the total number of labeling rules a food industry professional must follow. Food and Drug Administration FDA has announced the availability of a Small Entity Compliance Guide SECG to help packaged food manufacturers meet federal standards in the final rule Food Labeling. Last Updated March 10 2020 104 PM.
Pure Food and Drugs Act. The deadline to meet new FDA requirements is January 1 2020 and the deadline is. A The following foods are exempt from compliance with the requirements of section 403 i 2.
Revision of the Nutrition and Supplement Facts Labeling DATES. For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR. At the beginning of this year the Food and Drug.
The announcement of the guidance is published in the Federal Register on February 4 2020. RISK OF THYROID C-CELL TUMORS. Your Guide To The FDAs New Food Label.