Meningococcal Conjugate Vaccine MCV4. Meningococcal disease is devastating and debilitating with a staggering 1015 case fatality rate.
Meningococcal Vaccine A C Z 1 Dose
Vaccines can help prevent meningococcal disease which is any type of illness caused by Neisseria meningitidis bacteria.

Meningococcal vaccine mcv4. However similar to the polysaccharide vaccine against pneumococcal disease it is most effective in adults and does not consistently generate immunity in young children. Booster dose at age 16 years. For aged 2 through 55 years Indications for Use and Schedule Routinely administer.
Persons aged 56 years or older who have never received a meningococcal vaccine and anticipate needing only on dose MPSV4 meningococcal polysaccharide vaccine is preferred Persons who inadvertently receive MPSV4 should be revaccinated with MCV4 using a minimum interval of 8 weeks. A conjugate vaccine of meningococcal polysaccharide used for active immunization against invasive meningococcal disease caused by Neisseria meningiditis serogroups A C Y and W-135. There are 2 types of meningococcal vaccines available in the United States.
We conducted a vaccine response study among 67 ad. The MenB vaccine for people age 10 years and older who have certain health conditions or are in an area with an outbreak of serogroup B meningococcal disease Why are meningococcal vaccines important. Immunization with the conjugated quadrivalent serogroups A C Y and W-135 meningococcal vaccine MCV4 after hematopoietic cell transplantation HCT is recommended.
It is recommended by the ACIP for children 11 to 12 years of age college freshmen living in dormitories and other high-risk populations. Meningococcal conjugate vaccine MCV4 for ages 2 through 55 Meningococcal polysaccharide vaccine MPSV4 for ages 2 and older including older than 55 Both vaccines can prevent 4 types of meningococcal disease. 13 At Sanofi Pasteur we believe in a world where no one should suffer or die from this vaccine.
The meningococcal conjugate vaccine or MCV4 was approved in 2005. It uses antigens taken from the polysaccharide capsule and then bound to. In May 2005CDCs Advisory Committee on Immunization Practices ACIP published its recommendation to vaccinate all 1112 year olds with MCV4.
In 2006 only 117 of adolescents. However immune responses to MCV4 have not been prospectively studied after HCT. There are two kinds in the US.
Meningococcal conjugate MenACWY vaccines Mentactra and Menveo Serogroup B meningococcal MenB vaccines Bexsero and Trumenba Learn more below about which of these vaccines are recommended for adolescents adults and infants and children. The first conjugate meningococcal vaccine in the United States MCV4 Menactra was licensed in. Meningococcal conjugate or MenACWY vaccines Menactra and Menveo Serogroup B meningococcal or MenB vaccines Bexsero and Trumenba.
For aged 9 months through 55 years MENVEO Novartis. MCV4 is as effective and as safe as the older MPSV4. 1 Fortunately most cases can be prevented through vaccination.
Teens who receive their first dose at age 13 through 15 years should receive a booster at 16 through 18 years or up to 5 years after their first dose. Meningococcal disease can cause meningitis infection of the lining of the brain and spinal cord and infections of the blood. Even when it is treated meningococcal disease kills 10 to 15.
With rare exception it was not interchangeable with MenACWY conjugate vaccines. MCV4 Menactra MENVEO Meningococcal Conjugate Vaccines MCV4 Age Indications for MCV4 Vaccines Menactra sanofi pasteur. Meningococcal ACWY vaccine can help protect against meningococcal disease caused by serogroups A C W and Y.
A discontinued meningococcal polysaccharide vaccine MPSV4 Menomune Sanofi Pasteur was available in the United States until all doses expired in September 2017. Meningococcal disease is rare but people do get it and teens young adults and people with certain health conditions are at increased risk. There are 2 types of meningococcal vaccines available in the United States.
- One dose at aged 11-12 years. A meningococcal polysaccharide vaccine has been available since the 1970s. Meningococcal conjugate vaccines MCV4 Preteens should be routinely immunized at 11 through 12 years of age and given a booster at 16 years of age.
Meningococcal meningitis is a rare but potentially devastating disease which can strike anyone anywhere in the world. MCV4 was developed to prevent meningococcal disease resulting from infection with serogroups A CW or Y. A different meningococcal vaccine is available that can help protect against serogroup B.
![]() Meningococcal Vaccine Diseases And Conditions Pediatric Oncall
Meningococcal Vaccine Diseases And Conditions Pediatric Oncall
Https Www Paho Org Immunization Toolkit Wp Content Uploads 2017 05 Chapter13 Meningococcal Disease Pdf
 Selected Clinical Trials Of Meningococcal Conjugate Vaccine Mcv4 And Download Table
Selected Clinical Trials Of Meningococcal Conjugate Vaccine Mcv4 And Download Table
 Meningococcal Vaccine Side Effects
Meningococcal Vaccine Side Effects
 Green Hills Pediatric Associates Meningitis Vaccines
Green Hills Pediatric Associates Meningitis Vaccines
Https Www Maine Gov Dhhs Mecdc Infectious Disease Immunization Providers Communications 2016 April Meningococcal Vaccine Fact Sheet Pdf
 Suggested Recommendations Of Use Of Meningococcal Vaccine In South Africa Download Scientific Diagram
Suggested Recommendations Of Use Of Meningococcal Vaccine In South Africa Download Scientific Diagram
 Meningococcal Polysaccharide Diphtheria Toxoid Conjugate Vaccine Packaging Size 0 5 Ml Rs 3400 Vial Id 19637091562
Meningococcal Polysaccharide Diphtheria Toxoid Conjugate Vaccine Packaging Size 0 5 Ml Rs 3400 Vial Id 19637091562
 Sanofi Pasteur 589 05 Mckesson Medical Surgical
Sanofi Pasteur 589 05 Mckesson Medical Surgical
 Meningococcal Vaccine Page 1 Line 17qq Com
Meningococcal Vaccine Page 1 Line 17qq Com
 Update On Prevention Of Meningococcal Disease Focus On Tetravalent Meningococcal Conjugate Vaccine Semantic Scholar
Update On Prevention Of Meningococcal Disease Focus On Tetravalent Meningococcal Conjugate Vaccine Semantic Scholar
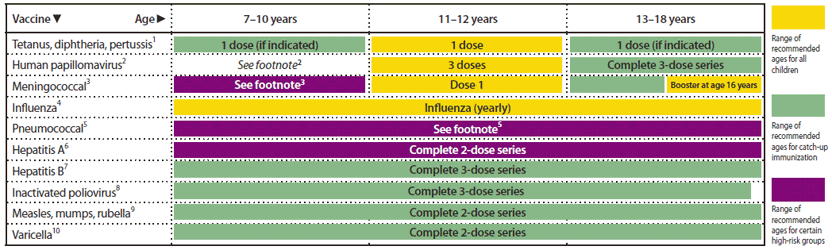 Recommended Immunization Schedules For Persons Aged 0 Through 18 Years United States 2012
Recommended Immunization Schedules For Persons Aged 0 Through 18 Years United States 2012
Https Www Paho Org Immunization Toolkit Wp Content Uploads 2017 05 Chapter13 Meningococcal Disease Pdf

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.